Dr. Joshua Bederson, Chair of Neurosurgery at the Mount Sinai Health System, presents the story of Mount Sinai BioDesign, a venture that is translating great ideas into viable products, and transforming the way we care for our patients. He also describes the plans for Sinai BioDesign's future as it grows under new leadership.
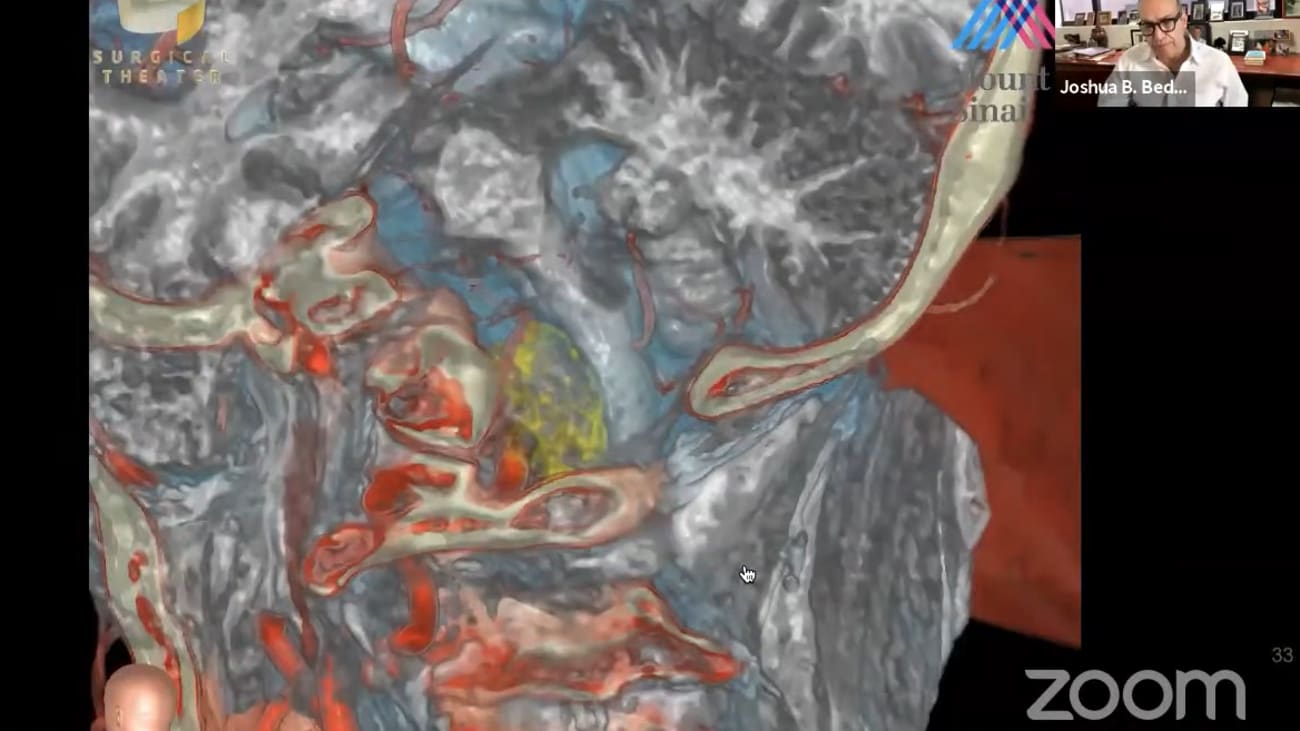
free for residents, fellows and medical students. Here is the information. If you have questions about this, you can email Lissa and she will send this to you regarding negotiations. Yeah. Good morning everybody dr Patterson needs no introduction of this group but this presentation will stand alone in the future on on youtube. So I introduced him to an unknown future audience. Dr patterson. Dr Joshua. Pettersson is the Leonard malice and korean joseph Graber, Professor of system chair of Mount Sinai health Systems department of neurosurgery. He's a skull base and super vascular neurosurgeon and his leader in developing advanced inter operative applications of digital visualized visualization technologies. He's led them outside under a certain department to become one of the fastest growing and increasingly respected nursery programs in the nation. He serves as co director of the Skull Base Surgery Center and Pituitary Care and Research Center and has performed more than 4000 neurosurgical operations at Mount Sinai. Dr Patterson is also a leader and innovator in neurosurgical technology development, leading the development of Mount Sinai's neurosurgeon simulation core. Ai Sinai and the topic of today's discussion Sinai bio design SAN about design is a highly innovative group that works with clinicians and scientists to help them develop and realize their ideas for medical devices from concept of patent prototype and even company development. I think this is one of the most exciting things happening in neurosurgery and even academia today. Thank you Dr Patterson thank you chris I'm going to share my scream. Mhm. Uh huh. Sorry about the delay here. Yeah. Yeah and you should just see about all of it now. Okay. Okay that's perfect. Great thank you. Good. Well today's talk is going to be focused on SAN about design a project that is designed to translate our domain expertise into commercially relevant innovations. Uh my disclosures have financial relationships with brain lab, surgical theatres, ice and you're a technologies investment investors which include companies like synchronous, but I want to say from the start that I have no financial role or, or gain from anything related to saN about design whatsoever. Nor are there any plans for that to happen in the future? It's been a real ride. Uh, working at Mount Sinai, which has changed over the years. It's an amazing place as you know, Uh, and with 42,000 employees, Mount Mount Sinai's new york's largest private employer. So it's a big, it's a big institution. Um, I'm the 8th chair. It's an old old department uh, for neurosurgery standards since 1914. And as you, many of, you know, we are now entering our 75th year of the residency program. The faculty has grown and as you can see, we have a large and diverse faculty, uh, very, very talented individuals, as well as a growing and incredible faculty of advanced practice providers. We're up to more than 50 faculty. We have more than 25 trainees. A couple of 100 neurosurgery staff were performing 5 6000 operations each year in 20 operating rooms every single day. And the department is improving at least as far as our ranking and our appreciation by peers. And here you see the U. S. News and World Report, hopefully Pointing us towards top 10 in the future. But equally important is what we're doing in research. Um And although ranking is not everything, I certainly do look at it. These are the blue ridge ranks for federal funding in research. And as you can see, we have now reached number one in new york state. We still have a way to go nationwide, but I have no doubt that with current efforts of our scientists this will continue to improve neurosurgery is a very diverse field. Uh cerebral vascular disease. Skull base primary and malignant brain tumors, deep brain stimulation for movement disorders and psychiatric disease. Pituitary, neuroendocrine epilepsy, spine, pediatrics, trauma, you're a critical care. Each one of these has different from the others as geriatrics is from G Y N. But one of our most important services is the fact that we have access to the brain that allows us to facilitate science and discovery. And in research, I think many of you are familiar with some of our most successful programs linking brain access to science here. You see brian couple and Alex Charney's living brain project designed to relate human subjects has neuroscience tools. This is a very exciting project that has led to new grants for both departments and new discoveries. I likewise see act with Helen neighbor is a prime example of how brain access promotes science and science promotes discovery. Uh Saudi ted responsive neuro stimulation leading to discovery and probably best exemplified by the vascular program. As you heard. Tom mentioned. One of the reasons he's here is the vascular program led by J. Marco professor and senior system vice chair. Um J is a world leader in several different realms. Um He's a publishing powerhouse and one of his, one of the many things in his background is his time at Buffalo, where the Jacobs Institute is and that is a medical innovation center that has developed under Nick Hopkins over the years, jay's motto is that care improves research improves care. Um And he has used the access to the brain to develop his role as a clinical trials, expert internationally, not just in the number of cases and number of clinical trials were enrolled in, but also in our role in those trials. As often the number one N roller in the trials that he participates in This together with our other researchers has led to a very busy clinical trials office in neurosurgery with 25 full time employees right now. Um and this has also led to major revenues coming in from industry. Many of the much of the finance is coming into the research infrastructure have been through industry. Um and this is reflected in a paper recently published Showing that the neurosurgeon department currently ranks number four in the United States in industry payments related to research. And if you look at the other places, one is fine, one is tumor and the only other one that is vascular sparrow. So really we're number two in the country relating to vascular neurosurgery. Uh so clearly this program of ours that focuses on discovery and research is important. What about commercialization? And that's where Sun I bow design comes in, which I view this program as Mount Sinai's main Center for MedTech Innovation. Uh Other than as opposed to research, this is a med tech device focused program whose goal ultimately will be to generate commercial assets for the Department of Mount Sinai with an embedded innovation program. And I wanted to give you a little background on how we came into being and where we're going to go. Um I started out as a suppose that academic neurosurgeon. I joined Mount Sinai in 1992. It took me 10 years to get my first R. 01 S. P. I. And was the first neurosurgeon to do that at Mount Sinai. But that was a long slog starting with an American Heart Association grant and our 29 Which was the equivalent of today's are 21 but lasted five years. And then finally the R. 01. Um As as my vascular program and skull base clinical program grew, I became more interested in three D. Modeling for preoperative planning. Um and in about 2008 we began a program called the Simulation Program. We develop collaborations with the Canadian Research Council and in 2013 recruited uh- Anthony cost to actually Pat Covic recruited Anthony into the scientific computing world. But Anthony and I rapidly became collaborators and friends And we were fortunate enough that he moved into the department full time in 2015. At the time we called ourselves the neurosurgery sink or and our mission was basically to bring simulation into the general workflow of neurosurgery every single day. And this is an example. Some have you seen this example? But I like it. It's very illustrative. This was a 71 year old man presented with a taxi to this was the tumor meningioma of his ventral cervical maxillary junction. And this is what the simulation shows. Ah the classic workflow is that we will simulate and plan the case. And here you see how we focus on tumor, blood vessel and brain as a first pass and then we can overlay soft tissues obtained from a co registered MRI scan to finalize our plan for surgery. So this is a fairly classic planning scenario. Over time this became almost routine and we have now defined what I think is the fields standard uh defining preparation, planning and practice of the A. R. V. R. Heads up display workflow neurosurgery. Um And we have published this, we have demonstrated this throughout throughout the world. Uh It's interesting to note that Anthony's expertise is in segmentation, automated segmentation so that uh the computers basically can figure out what's important and select the most important areas of the image to model. Yeah. So that was that case. This is a current case. So that was 5, 10 years, five years ago. The case I just showed you this is a case from a couple of weeks ago and those of you who are on the blue service will remember this patient also presented the same way and here's what the simulations currently look like again. Uh This one focuses on soft tissue and tumor and then we can overlay bone and blood vessel. And what this demonstrates is that it's likely to be a challenging case because the vertebral basilar junction is displaced superior early by this tumor and brain stem is draped over the back of it at surgery. And this is from the case just a couple of weeks ago, you see the brain stem door slowly displaced the tumor peeking out and our window between branches of the nerves and blood vessels is very narrow. It's very helpful I think to know with this heads up display how far lateral this tumor goes to the vertebral artery, but also how far we have to reach in order for us to get to the VB junction, which after a little bit of training, you begin to be able to interpret these heads up, display images over time. We realized that heads up display a AR and VR are useful and applicable in many different parts of the patient engagement profile. From initial consultation and planning, setup, patient positioning and so on and so forth. Uh huh. Not, not unimportant lee. Uh It's become extremely useful in our consultations with patients and families that they will understand better, what it is we need to do. one of the things that became obvious early on is that our needs in the operating room, our industry drivers and the reason I knew this is that before long without really trying, I had the leaders of certain key industries in my operating room and develop relationships with with all of these people who many of whom I now call friends. Stefan will smile modi avatar, Lon gary, dirk, bruner Cameron, P. Iran and and J. Macos relationship with tim scandal also brought him into the fold to a certain extent. Um and these relationships allow us to effectively help them develop their technologies to meet our needs. Um So getting back to what Anthony was doing, he was working on automated segmentation and early on he and his students, Solomon Samani and Doug Yunus, an orthopedic surgeon um realize that if they could automatically segment a diseased hip and have our modeling center called the Rapid Prototyping center three D. Print uh an implant. They might be able to form a company which they did. There's a kind of an inherent problem with some of the implants. You know, they're really one size fits none that aren't really designed to fit any one particular patient. A lot of um orthopedics is a medical technology company in the orthopedic space. What we're developing is the next generation three D. Printed implant that is optimized for press fit fixation and insertion with the state of the art orthopedic robot. The line. So this this was our first company out of SAN about design. Uh huh. It's Now valued at about 75 million and has returned over $4 million dollars in equity to Mount Sinai on a series evaluation. So if we can influence industry and create companies can we also develop our own devices. And that that really was the question. Um around that time the rapid prototyping center became available and after a pitch to Dr charney, he gave it to us. We worked with scott Friedman and Joel Dudley to transition to entirely to neurosurgery, the rapid prototyping center. And as tom Oxley and his talk just before mentioned early funding was key and there was no other place for that funding other than neurosurgery. And so uh I told the dean that I wanted to invest in this concept of a device innovation center. And and he said yes it was it was money generated from neurosurgery but I also needed his spine and his permission and we got that. And so over the past several years we've invested heavily in this in this program. However, the first step was to prototype the process of developing and faculty facing needs based innovation program and I had to pick a problem to address. Um And so the one that I was interested in is the concept that a deep electrode, such as one that you would use in scG, although it provides very valuable information loses information that is generated on the cortical surface. However, a cortical surface electrode, at least in harry up until recently required a large opening. And you can see that both Tom's company and this concept are again going after something similar, the information electrical information available on the cortical surface. So I went back to my own drawing board Um and in 2016 made some drawings about an idea for a device that would allow us to drill a slanted burr hole and ultimately allow us to insert a subdural electrodes At the bedside. And you can see the 2016 timeline here. And this is this is the borough hall that I had hoped our device would eventually create. Who took it to an artist mom. And we called it the minimally invasive cranial access device and then I had to prototype the device. So I went to my home studio and home depot and through this together, which I still have kicking around my office somewhere. And this is the initial rude crude prototype. Uh huh. We then brought that down to saN about design and prototyping center converted the drawings to CAD. Um And then in the RPC Created a three d. Print um that then holds the modest rex drill. Yeah. The next phase was to collaborate with Montana Innovation Partners to do a search of the patents out there and we found that really there was nothing out there. Um We also did a market assessment with mm sip ah the preparation for the patent and the filing of the patent. So what is M. S. I. P. Um for those of, you don't know, Mount Sinai Innovation Partners is run by eric liam. There's an extensive staff um and this is uh in other places this might be called the Med Tech Center for Mount Sinai. It's been extremely valuable to develop this relationship. We have a coordinated strategy, particularly now that Phil Williams has joined us in which we discuss our strategy for commercialization very early in the process of developing new ideas and we interact back and forth with their support each step of the way. One of the most important parts of Mount Sinai bow design is the embedding of SBD in our operating rooms. And here you see the Sanibel design on a On the 8th floor uh adjacent to ours and down on the M. C. Level so that we are really truly colossal equated with the surgeons doing our our activities. We have a structured, fairly routine process. We identify the need which is really the problem that each surgeon faces every single day. How many times are you in the operating room thinking if only I had an instrument that could turn left or right or give me a different view. So you have problems that you face every day that need a technical solution. The idea is that we bring these problems to sun about design and it's the son of bio design engineers that worked with surgeons to identify potential solutions which are then modeled and prototype within bio design. And since we're co located we provide immediate feedback to the surgeon and then move on to translational development while iterating with sip The idea is to generate commercial assets whether through grants, licensing or startup companies. And we're making progress. We currently have uh 15 active projects and here you see our portfolio right now and I'll describe some of these, um, you can see the various phases of discovery commercialization development uh, and where the, what the commercial approaches, whether through a grant, a startup or license and you can see where we stand on each one of these. I want to give you a couple of examples. Yeah. Um, Since 2018 though we've had 75 faculty engagements, uh, 17 patents, 26 tech disclosures where one, we've got one startup that's developing, we've got three grants applied for uh and one success, commercial success so far. These are our top five programs right now, chill DBC valves, the pharynx s. Operator and micro print a chill is an intracranial cooling catheter. And this project physician leaders chris Kellner um as you know, chris was recruited in part to develop our program for inter cerebral hemorrhage management and uh he has developed a technique and technology that both evacuates and can cool the catheter to reduce the peril. Additional brain damage, cooling is a proven protection strategy both in heart and in brain. Although cooling the brain has become extremely difficult and this is a device designed to cool a portion of the brain around an inter cerebral hemorrhage cavity who's hemorrhage has been evacuated. Um Yeah this concept has been vetted through deployment in each phase of the process. Sergio scope removal, tunneling attachment cooling, placing the fact that we can generate models in the basement ah potentially deploy them in the operating room Or test them on the 26th floor is a huge advantage of having SAN about design in one building together with neurosurgery. Um and where we stand right now is that the patents have been obtained? We've got a large animal trial in preparation. The grant was submitted um and you see that this is a very well thought out process with the team up there. This is what was described to the NIH and what you see here is our plan for five years that ultimately hopes to spin out a company and this is what we described to the NIH D. B. C. The detachment balloon catheter originates with Alejandro. Um Also we have the patents up and running um and here we are actually hoping to form a company around this. Um we are in active negotiations to recruit a ceo for that company. This is what the device looks like and it's goal is to be the first euro detachable balloon catheter uh in this space, right? The next generation valve project As one that tom is leading And this is designed to solve the problem of meeting two hands to detach blood loss and complications related to detachment of the valve. Um And likewise we're pretty far along. This one actually may go fastest of all of them, lumen is another one of chris's. This is one of the most interesting ones I think and the concept is for near infrared light and the treatment of neurodegenerative and other brain disorders. Um as well as to form a company, we don't just live on ourselves to neurosurgery, although that is clearly our focus. We would like to work with other departments and we're doing this with cardiac surgery here with the micro print designed to since pressures, closing pressures in the mitral valve um as well as working with E. N. T. On the pharynx aspirated. Er And this is a project of J. Marks who is really integrated nicely with the program. This is also rapidly going and we're currently speaking with several different companies about a licensing or royalty agreement for this project. Um So I think that we have, we've gone from prototyping to demonstrating we can partner with physician creators and here you see some quotes from our physician creators. Um We do more than that. We also are transforming students into leaders. Uh The M. O. Of this whole project is that we're going to take students assign them two projects. They will learn the process as they develop those projects and ultimately they will graduate as ceo of a company around that project. And this is something that's taken primarily from stanford bio design. And here you can see some of our students um and turner baker is actually one of our main faculty and leaders in Sana bio design. Yeah. So where are we going from here? The most important thing is that we intend and need to expand our team. It's a relatively small team right now, with way too many projects and far too much work that can be done by such a small team. We're going to expand the design fellowship, enlarged the resource pipeline. We hope to integrate a sauna separate arm of SAN. About designed into the program. We're gonna work on community outreach and enhancing the visibility of SAN about design. Yeah, So how do we expand when we're losing key faculty and many of, you know, by now that Anthony Costa left on Sinai, he was recruited by NVIDIA to lead their ai driven healthcare strategy. It's a testament to Anthony's talent that he was recruited, but also of the Sinai bow design concept that he was able to grow as a leader of Sunai bio design and I think it's nothing but a great success. We remain really close with Anthony and hope to continue our collaborations with him as time passes. Um this is a sign of our design team right now. Um it's led by Ben Rappaport and Tom Oxley whose outstanding presentation you just heard, I hope that he stays available enough to be a vital member of SAN about design. Over time Phil Williams has been a welcome addition and an essential edition directing our business development as a member of M. S. I. P. Turner baker and Alexis are very strong members and then other members of the neurosurgery department, students who are critical to SAN about design and here you see people who have passed in and out of SAN about design but who remain extremely important wow! Ben Rappaport is the new director. Uh those of you who know him already know that he got his PhD in electrical engineering from MIT, his medical degree from Harvard um and he's completed fellowships an endovascular as well as endoscopic skull base surgery. Um He really packs a punch. He's a med tech entrepreneur, he's a great leader, he's a scientist. Henry is a marathon runner with multiple sub 3-hour marathon. So there's your resilience right there. I think Ben has everything that he will need to take san about designed to the next level and really welcome welcome him. Um and I mentioned how valuable tom has been in, giving us his perspectives that he has gained from forming sink Ron turner baker and Alexey are incredibly important members. They're both project managers, basically overseeing the full process, but they have way too much on their plate. Um And so our plans are to expand our fellowship um to leverage our network throughout new york and we have partnerships with many different programs. Um We're going to be bringing in ah tech companies from around the world to get first looks at some of our technology and give them an option to participate. And we have already developed more of our modeling program. Initially we we're doing courses at places like double and S. And we needed three D. Printed skulls and brains. And so we did our own, we then realized that this is actually a commercially valuable product which we are now making um we call it brain case um and it is both the shell and the soft brain that can be used for simulation and modeling. Um And you can see we're using it here for SCG and we hope to use it for ah I. Ch. And other disease processes. We're gonna be working on social media and communications um and partnering with the Department of Education. Uh We see potential for collaborations with the biomedical engineering institute. Zahi and pretty uh have offered to partner with us and we see numerous opportunities there. So this brings us back to where I started. You know, none of this is useful unless it can help us with the technologies and techniques and going from these devices. I showed you to the ability to remove this tumor. And here you see the final result with the vertebral basilar junction as well as the origin of the anterior spinal artery and the desired outcome with the patient that's still a long way off. Um And the whole goal of this is to generate devices that make money for the department but also solve relevant problems for our patients. Um I started by saying that the doctor Charney had supported us in the beginning and he continues to support he's seen what we have done and is committed additional support to further this process. So I am sure that the future of SAN about design is bright but as Tom said and I learned so much from his lecture, I'm not sure how you put it but a continued sense of urgency. I'll have to get the quote better from you Tom and that's really where we are now, is to maintain our momentum and to actually accelerate with new students a prototyping fund uh and to get additional space. So I hope that I have given you a sense of how brain access for science and discovery is what drives this one of our service lines in saN about design. Um it's certainly exciting to be part of this and I hope that in the next five years I can give you even better follow up as to how far we've come and what we've done, so I think I'll stop there and take any questions if if you have any. Mhm. Yeah, I finished a little earlier than plan but um let's see, are there any questions in the audience? I do see some questions, hold on, I'm I'm activating some questions here. I think I just make a quick comment doctor medicine that I think what's really and Ben has spoken about this as well, that we really think there's an opportunity to grow the medical device um community in new york city and um you know, control labs recently sold to facebook, there's this sort of resurgence of tech and hopefully now MedTech as well, that's happening in new york city right now. And we've taken lessons from how stanford were very successful with their program. And Ben's got some ideas on how to bring that through in new york that your support of the next few years and then growing out in the new york city infrastructure means that everything is in place for this to be hugely successful. And I think it's really exciting where it could go. Yeah, I hope so. Then you want to comment a little bit on what you see as the future of sun, about design. Absolutely tremendously, tremendously exciting. Time for sign about design and Mountain North surgery. And I'm thrilled to be joining the team in this capacity. So thank you and it's great working with tom and with the other members of the group and and I would just say that that some of the key factors to success in the past and president future, our our ability to engage with all of you and other colleagues at Mount Sinai with um you know with promising ideas and uh with students and others in the new york city area and and really if you if you have ideas or want to think about even engaging, um you know, sign of iodine has has a lot of resources and talent to make make ideas reality over time. So really I welcome, I welcome those conversations over time and and please please be in touch. This is a really exciting time I come from the body. I'm sorry, I just wanted to just wanted to tell everyone that I was watching what I think was one of the most exciting times for bio design was when and J. Marks probably not here but it's an E. N. T. Physician who was working with I think it was Alexey and Anthony And it was during COVID and there was a meeting that happened in bio design. They just came through, they spent about 10 minutes, they talked about this issue of viral particles during intubation and came up with this very quick and simple idea of this aspirated device which was one of the comments. And then Alexi was there and suddenly to grab the idea from the position and then turned it into an idea and kept the momentum going. And I think that's the beauty of it. If anyone's got concepts or ideas swing past, have a quick conversation and it's an infrastructure to carry on your ideas because positions are too busy to carry them forward. And so that's the interaction that works. And I just thought that example was just, you know, everything perfectly coming together. I think that's that's really important. Which is that, you know, as surgeons were natural innovators. I mean, Dr Pedersen mentioned this during the presentation. How many times you hear yourself or a colleague say, I wish I wish I had this or that device or capability happens all the time. And uh we we live basically within the department and we have rapid prototyping capabilities and very talented engineers and we already have a track record of um prototyping and putting out devices that actually work. So this is a way of sharing your ideas in a very exciting way, working with people who have the ability and proven track record of um, you know, making prototypes and actual working devices. So please come share your ideas and then I think you'll feel the excitement as you sort of make a concept that you've been thinking about into something that is testable in a prototype, um and and closer and closer to a real workable device. one of the things I wanted to say was that, um, although members of the team are not here to make money off of these devices, uh if you bring an idea, inventors actually can and do retain all of the rights that any inventor would developing an idea through Mount Sinai and there are revenue streams that come back when eventually this and and when and if these things do become successful. Um another question that came uh was that in the context of limited resources with so many different projects, how do we prioritize these projects? And what is the process that we you spend out? Do you want to just talk about what the processes for prioritizing? So thank you, Ben. Before you answer, I I asked that question and I wanted to congratulate the team for this incredible enterprise that you have together and I do understand that it's important to come up with ideas and to test as much as possible. I also want to take the exact opposite perspective uh In our world, resources are limited, so maybe talking a little bit about how to prioritize and what are the strategies that revolves around that? Thank you. Yeah, sure. Thanks for the context there. Um the group is called bio designed for for a reason and that is that over the last 20 years, this concept of bio design, which was really pioneered at stanford um has been developed as a pretty disciplined biomedical engineering um procedure and um it's actually been codified now, it's a textbook in its third edition called bio design, um that came out of stanford uh standards by Design program. And the process begins with evaluating a new idea, ideation, understanding what the market needs are and really staying true to clinical needs. Uh And um it's a maybe a subject for a slightly longer discussion, but we really try to deeply understand what the clinical needs are, what the market needs are, what is feasible, um And and then um through that to find the means to make ideas that meet those thresholds of um of feasibility and clinical need uh into a reality. We tend not to shut down projects because of lack of uh you know, lack of means, you know, when there is a project that meets the needs that meets the threshold of uh sort of medical necessity, urgency, piece ability, market meat and so on. The the imperative within the group is to bring those projects forward. And we find we have found ways through grant funding, philanthropy, industry support and so on. Um So it's it's it's uh it's limited only somewhat by personnel and uh and and funding and we hope certainly to grow those um aspects as more uh more great ideas and concepts come into the group. And you you saw already, I would say that at this time we have about six or seven very mature projects within the group and uh we're actively working to expand the abilities of the group to meet those as the number of great projects uh rises. So I just want to emphasize, we're not here to shut down projects were here too to make the good ones possible. Have a question. Mhm. Yeah, go ahead. 10 beer. Um First of all, those are two very inspiring presentations and it's an incredible opportunity. We have to have bio design of the department and I think uh many of us have ideas and may have not taken full advantage and I think it's very inspirational. My question had to do tom had a question about sort of the intersection between being involved in industry and academics and he sort of answered it from how being involved in, I mean, involved in clinical things and that the clinical thing could help perhaps his business side. I'm wondering about the reverse to any of the people either dr Pedersen oxy or a rapper part. How does involvement in industry in the potential for payment or conflict of interests affect academics and your ability to publish, present and maintain your academic profile? I'll take a stab at this. Um It definitely does. And there has to be a management program for each project that you undertake um has to be listed. It has to be declared. Uh And when you have a relationship with a company, let's say, or an industry, you cannot really be involved in the research or data analysis of that project. Um So it definitely has an impact. However, over the past several years, I believe that Sinai has become a little more open to working with us on these management plans. Sometimes they look at what we're doing and challenge, but when we explain what the relationship is and how we've managed it, they've been fairly understanding. Um but you can't, you know, if you have, you know, for example, with with Brain Lab, I would love to be able to do a study in which I'm, you know, lead investigator on some of this heads up display and so on and so forth. But since they are funding some of the work can't do it, just can't do it. So it has an impact. Uh does anyone else want to comment tom do you want to comment on that one? Yeah, I was just thinking through the experience I've had with peer review in uh you know, high impact journals, I think When the disclosure the disclosures have to be up front, but I think there are examples where companies have um still published in high impact journals because the data has has stood up on its own two ft. But I think the the rigorous the level of rigor that the peer reviewers put you under when you have those disclosures is significantly higher than if you did not. Um but you know, google, I'm thinking of google brain, I think Deepmind. Um there's been a bunch of really impressive pieces published by companies. Um, so I think it's possible. I just, I think from an academic point of view, yeah, the disclosures have to be up front and then you're held to it, you're held to the torch more than you might otherwise be. Although I think as we've gone on, I've noticed this increasingly, um has, you know, I think the ability to do this continually reduces. So I think if you're taking the decision to pursue a commercial activity, your ability to focus and be a pure academic diminishes over time. And I've noticed that happening, I think I could add one thing to that because I think this is a really important topic and there's sort of two questions here. One is um, how do we maintain the ethics of the scientific process around um, what happens when a device is either motivated by or used to treat patients? But there's also a commercial interest involved. And I think there's sort of a car leather question which is when you have position innovators or academic innovators who want to make their ideas a reality? How do we respect there? Uh, their intellectual contribution in the financial way as the, as that concept becomes a reality? And those both of those are the subject of a lot of discussion over time. But I think here at Mount Sinai and in bio design and in our surgery department, we have a lot of people, many of whom are on on this on the call today who have been able to successfully navigate that. And we're definitely committed to doing it. I think tom raises a great point, which is that increasingly there is a trend within industry in the tech industry to publish results in a peer reviewed in a peer reviewed way. I think that's definitely the case in the artificial intelligence community and and certainly in the bio design, we're committed to um to navigating that path of kind of ethical um ethical approach to all of these issues. And uh, I think that you're going to find that in in your interactions with us. Thanks tender. Hey, tom. There was another question from the audience on yours. Um are you, what operating system are you going to use? Are you Mac or PC? Uh and if you are Mac, can you get a pc driven device or vice versa? Yeah. Being recorded for Youtube. Uh Thank you. Um this is a this is a critical question that we're looking at. Currently, I would say the framework with which we're going to make that decision is around F. D A. And the challenge with FDA is that everything needs to be locked down. So it probably is the case that we're going to choose an operating system to start with. Um Our goal is to achieve what Cochlear have achieved. And that is to draw a line with where the Class three implantable becomes a Class two. And then when you're dealing with the Class two component of your device, you can make iterations more quickly. Very hard to do that with the Class three. Cochlear recently achieved this by and this was only about a year ago, they Um achieved this when the Bluetooth left the cochlear, they considered that they got that regulated as a class two. And they had their first patient there. Cochlear connected directly into the iPhone. So um and the first patient to then speak to their wife could hear the voice very clearly through the iPhone, didn't need to touch the phone. I think it's an example of where these prostheses are headed. Um And but F. D. A. You know uh this is new for FDA. And so finding a way that we can iterate through software updates in a way that FDA finds acceptable is a major challenge for the entire industry moving forward and we're facing that now. But um I can't tell you which operating system we're going to use just yet. Great. Mhm. Okay. Are there any other questions or comments? If not? I think we can and that today's session. Okay. Thanks everyone. Happy to talk offline. Thanks tom.